Microphysiological Systems Lab
The MPS Lab is focused on the development of tissue engineering strategies for a variety of applications including:
- • regeneration / replacement of dead or diseased tissues
- • high throughput in vitro drug testing
- • improved understanding of the structure and function of healthy and diseased tissues
Using cells, biological and synthetic materials, nutrients, and growth factors, the MPS lab synthesizes tissues in vitro. Led Dr. Chris Heylman, this work draws on a wide range of disciplines including cell biology, polymer chemistry, materials science, mass transport, fluid mechanics, and microfabrication.
Current Projects
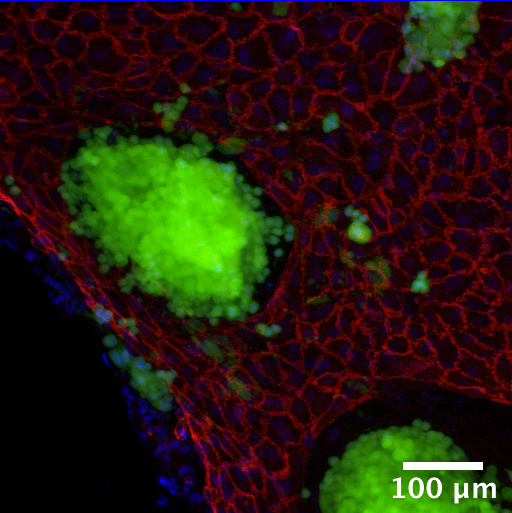
Colorectal Cancer Tumor Model
Establishing an in vitro three dimensional human colorectal tumor model and assessing model response to pharmaceutical compounds.
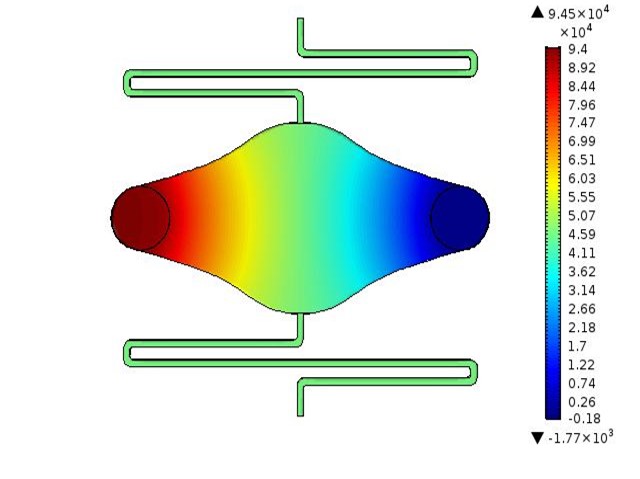
Microfluidic Devices for Tissue-on-a-chip Applications
Design and development of microfluidic devices for use in tissue-on-a-chip applications. Includes device design, COMSOL simulation, mold and chip fabrication, and chip validation.
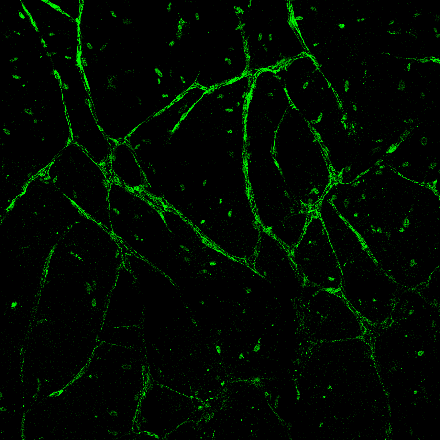
Injectable 3D Vessel Network
Developing an injectable solution of extracellular matrix proteins and human cells capable of forming a three dimensional network of blood vessels to support nutrient transport and waste removal within a tissue grown in vitro.
Want to get involved?
Interested in working with us to develop tissue-on-a-chip systems? The MPS Lab is always looking for curious and motivated undergraduate students to join the lab. To explore the lab and shadow current members please visit this MPS Lab Interest Site to learn more about different opportunities in the lab.
Please help support the MPS Lab with a one-time or recurring donation. Your donation directly supports the science and innovation of the MPS lab as well as the technical training and education of the next generation of scientists. Donations help fund supplies, equipment, and student travel to conferences.
